Concerns are mounting among users of some drugs subsidised by the National Health Fund (NHF).
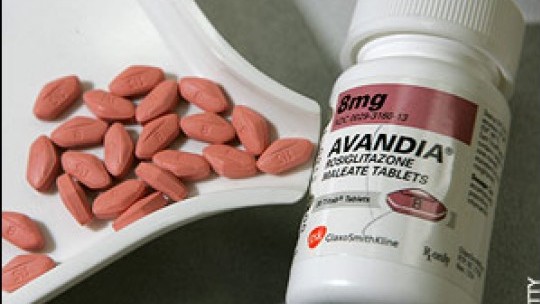
Safety data from clinical trials have shown that there is a potentially significant increase in the risk of heart attack and heart-related deaths in patients taking the diabetes drug, Avandia.
As a result, the marketing of the drug has been suspended in the European Union.
However, in the United States, the Food and Drug Administration has not banned or suspended the use of the drug, but has alerted users to consider taking alternative medicines.
The FDA has also placed additional safety labelling and restrictions on the product as it carries out a Risk Evaluation and Mitigation Strategy programme.
Norvac yanked
In the meantime, elderly persons who normally purchase the blood pressure drug Norvac discovered this week that it has been yanked from the National Health Fund's subsidised list.
As a result, persons who would normally pay about $40 will now have to fork out the retail price of $4,000.
The NHF says it will provide the reasons behind the decision in a release over the weekend.
MOH evaluating Avandia
In the meantime, in a release on Friday afternoon, the Ministry of Health says it is aware of the recommendation to suspend the use of the diabetes drug, Avandia and is now evaluating all the technical information available to make a decision whether it should discontinue its use in Jamaica.
It says according to the European Medicines Agency in a few months the drug will no longer be available to patients in Europe.
The Ministry says it will advise Jamaican health professionals and the public once a decision has been taken.









 All feeds
All feeds







