The US Food and Drug Administration announced on Wednesday that it would seek to pull a widely used ingredient in cough and cold medicines from the market, after the agency's scientists concluded that the oral version of the drug is ineffective as a nasal decongestant.
The FDA's proposal comes more than a year after the agency's outside advisers voted against continued use of the ingredient, called oral phenylephrine.
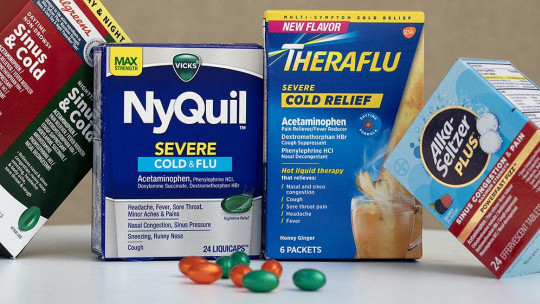
A number of common over-the-counter nasal decongestants have relied on phenylephrine alone or in combination with other ingredients for years, including some cold and cough versions of Advil, NyQuil, Tylenol and Theraflu.









 All feeds
All feeds







